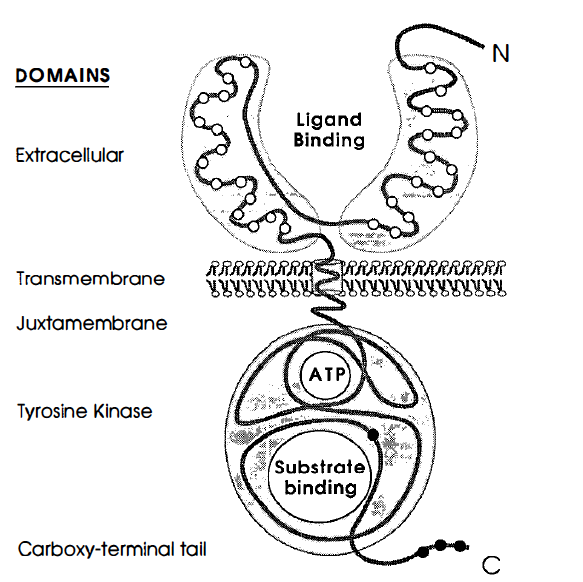
Figure 1. Prototypical structure of receptor tyrosine kinases and a look at the proteins different domains.The open circles represent
the cystein rich regions, and the closed circles represent the tyrosine residues. Image from Yarden and Ullrich 1988. Permission Pending.
What is EGFR– tyrosine kinase?
Epidermal growth factor receptor (EGFR)–tyrosine kinase is a cell surface receptor protein (Woodget, 1994), that belongs to a large family of receptor protein-tyrosine kinases (RPTKs). All RPTKs play a crucial role in cell differentiation and growth, and RPTKs can be found in all multcellular eukaryotic organisms (Woodgett 1994). RPTKs become activated when growth factors, or ligands bind to them, causing the receptor tyrosine kinases to catalyze the transfer of a phosphate from ATP to tyrosine residues on target proteins (Schlessinger 2000).
What is EGFR– tyrosine kinases structure?
EGFR–tyrosine kinase is a monovalent, type I transmembrane glycoprotein (Woodgett 1994). The protein's N–terminus resides outside the cell, and the whole protein only spans the membrane once. The entire length of the polypeptide chain consists of 1186 amino acids (Yarden et al. 1988). The membrane acts as a division point that divides the ligand-binding side and the cytoplasmic side. In the extracellular domain, there is a 621 amino acid hydrophilic ligand–binding domain that contains many N–linked glycolysation sites (Yarden et al. 1988). The ligand binding domain conists of 2 similar domains that are linked together by an "arm."Also, there are two cystein rich regions within the ligand binding domains in the extracellular domain. 23 hydrophobic amino acids make up the transmembrane domain.The juxtamembrane domain seperates the cytoplasmic surface of the plasma membrane from the tyrosine kinase domain, and here, a threonine residue is present that is a known target for the phosphorylation of protein kinase c (Yarden et al. 1988).The cytoplasmic domain portion contains 542 amino acids, and this is where the catalytic portion of the tyrosine kinase resides (Yarden et al. 1988).
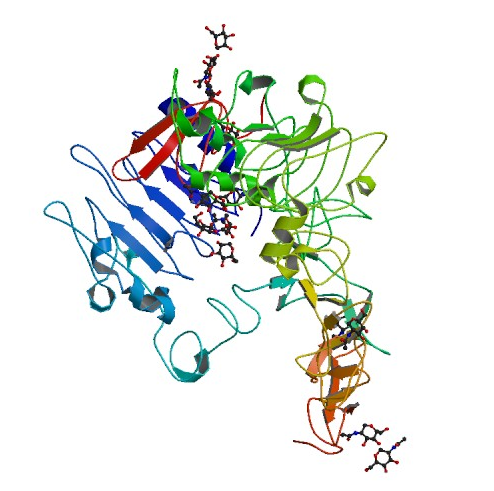
Figure 2. Structure of the extracellular domain of human EGFR in an inactive complex with EGF. Picture courtesy of PDB.
What is EGFR –tyrosine kinases function?
The function of EGFR–tyrosine kinase is to catalyze the transfer of a γ phosphate from an ATP molecule to tyrosine residues on target proteins. By performing this transfer, EGFR– tyrosine kinase activates other proteins that will send signal cascades to the nucleus. As a result, it uses a series of signal pathways to achieve its final goal; cell proliferation and division. For this to occur, however, the extracellular membrane first needs to bind with the proper ligand, which in the case of EGFR is epidermal growth factor (EGF), or transforming growth factor α ( TGFα). It is probable that EGF is bivalent towards EGFR-tyrosine kinase (Schlessinger 2000). A single ligand molecule is bound for a receptor dimer. When this occurs, the inactive monomer dimerizes as a result of the ligand induced conformation change (Woodget 1994). The dimerization of the receptor leads to intermolecular autophosphorylation. This results from the contact between the two cytoplasmic domains during dimerization, thus stimulating catalyctic activity that leads the two receptor molecules in a dimer to transphosphorylate each other (Woodgett 1994). The autophosphorylation of the tyrosine residues in the kinase domain activates the tyrosine kinase to phosphorylate the cytoplasmic substrates.The intermolecular autophosphorylation occurs at specific sites of the EGFR– tyrosine kinase outside of the catalytic domain, more precisely near the end of the c–terminus and the juxtamembrane(Yarden et al. 1988). The signaling pathway continues by phosphorylating, thus activating, the tyrosine residues of target proteins at the catalytic domain of the EGFR-tyrosine kinase (Schlessinger 2000). The target proteins recognize and bind to the phosophotyrosine binding sites that were created by the autophosporylation of the juxtamembrane and c– terminus (Woodgett 1994). The target proteins will then send signal cascades to the nucleus, giving it instructions for cell proliferation and differentiation.
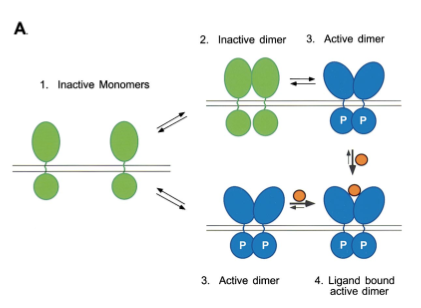
Figure 3. Ligand binding stabilizes the formation of activated dimers. The receptor dimers exist in a conformation that allows trans/autophosphorylation and stimulation of tyrosine kinase activity. Image from Schlessinger 2000. Permission Pending.
Additionally, EGFR–tyrosine kinase is capable of activating ErbB2 (another RPTK, in the EGFR family). The exact method for this signal transduction is only a proposition (Schlessinger 2000), but since no ligand has been found for ErbB2, it is hypothesized that the activation of EGFR–tyrosine kinase by binding to EFG may drive for the heterodimerization of ErbB2, thus forming a tetrameric complex with unoccupied homodimers of ErbB2 (Schlessinger 2000).
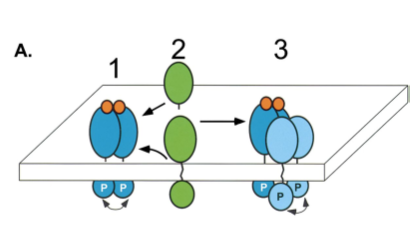
Figure 4. Activation of ErbB2 by heterotetramer formation as detailed in the last paragraph. Image from Schlessinger 2000. Permission Pending.
------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
References and works consulted
Woodgett JR, editor. Protein Kinases. New York: Oxford, 1994. 177-202.
Schlessinger J. 2000. Cell Signaling by Receptor Tyrosine Kinases. Cell; 103 (2): 211-221.
Yarden Y, Ullrich A .1988. Growth Factor Receptor Tyrosine Kinases.The Annual Review of Biochemistry; 57: 443-449.
If you have any questions or comments: E–mail huhayes@davidson.edu